In their letters, the FDA also said people using these unapproved eye products that claim to treat or cure certain conditions may cause delay or stoppage of treatments that are approved by the agency.
FDA sends warning letters to several companies, including CVS and Walgreens, over unapproved eye products."We will continue to investigate potentially harmful eye products and work to ensure violative products stay off store shelves so that consumers can continue taking the medicines they need without concern," the statement continued.
A list of the products was not listed on the FDA website, but a spokesperson told ABC News the products were named in the individual letters.claim to relieve redness, burning, watery discharge and feelings of grittiness.
Belgique Dernières Nouvelles, Belgique Actualités
Similar News:Vous pouvez également lire des articles d'actualité similaires à celui-ci que nous avons collectés auprès d'autres sources d'information.
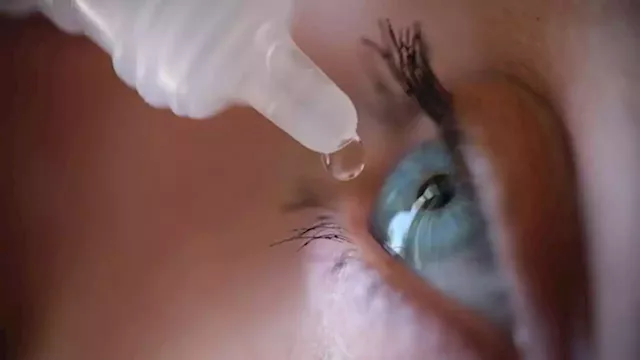 FDA sends letters to CVS, Walgreens, other companies about unapproved eye productsThe FDA sent warning letters to eight companies, including CVS and Walgreens, warning them against making or selling unapproved eye products.
FDA sends letters to CVS, Walgreens, other companies about unapproved eye productsThe FDA sent warning letters to eight companies, including CVS and Walgreens, warning them against making or selling unapproved eye products.
La source: 6abc - 🏆 250. / 63 Lire la suite »
