The U.S. government will stop distributing free doses of Merck & Co’s COVID-19 antiviral treatment molnupiravir by the middle of next month and expects it to be sold on the commercial market instead.
Merck, which developed the drug with Ridgeback Biotherapeutics, said in an e-mailed statement on Wednesday that it needs an updated letter of authorization from the U.S. Food and Drug Administration to allow it to start selling the drug commercially. It has taken a back seat to Paxlovid in the United States and the EU regulator recommended against the Merck drug’s use in the region.
It has also been linked to potentially transmissible mutations in the COVID-19 virus, according to a study published in the journal Nature last month. Merck said the study was limited and that it is confident in the clinical profile of the drug.
Belgique Dernières Nouvelles, Belgique Actualités
Similar News:Vous pouvez également lire des articles d'actualité similaires à celui-ci que nous avons collectés auprès d'autres sources d'information.
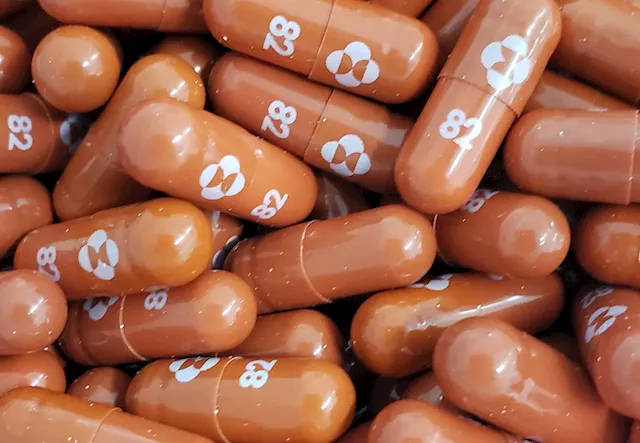 US plans shift of Merck COVID treatment to commercial marketBy Michael Erman (Reuters) - The U.S. government will stop distributing free doses of Merck & Co's COVID-19 antiviral treatment molnupiravir by the ...
US plans shift of Merck COVID treatment to commercial marketBy Michael Erman (Reuters) - The U.S. government will stop distributing free doses of Merck & Co's COVID-19 antiviral treatment molnupiravir by the ...
La source: SaltWire Network - 🏆 45. / 63 Lire la suite »
