Moderna said on Friday Canada's health regulator has approved its vaccine for respiratory syncytial virus in adults 60 years and older, making it the country's first authorized mRNA-based shot for the condition.Moderna said on Friday Canada's health regulator has approved its vaccine for respiratory syncytial virus, or RSV, in adults 60 years and older, making it the country's first authorized mRNA-based shot for the condition.
Earlier this week, British drugmaker GSK received approval in Canada for its RSV vaccine for adults aged between 50 and 59 years. GSK's Arexvy and Pfizer's shot Abrysvo, both protein-based vaccines, are also approved in Canada for adults aged 60 years and older.It also recommended adults 60 years and older get an RSV shot if they live in long-term care homes or other chronic care facilities.
Moderna's shot was approved in the United States in May for the same age group. It is also approved in Europe and Qatar."Public vaccine procurement in Canada involves many provincial, territorial and federal stakeholders. Moderna will work with all relevant stakeholders to enable eligible Canadians to have access to this vaccine," a spokesperson said.
The product does not appear to be listed in Health Canada's database of approved vaccines and the department did not immediately reply to CBC News on the status.
Belgique Dernières Nouvelles, Belgique Actualités
Similar News:Vous pouvez également lire des articles d'actualité similaires à celui-ci que nous avons collectés auprès d'autres sources d'information.
 Health Canada approves Moderna’s mRNA RSV vaccine, company saysAgency approves the vaccine to protect against respiratory syncytial virus in adults age 60 and older
Health Canada approves Moderna’s mRNA RSV vaccine, company saysAgency approves the vaccine to protect against respiratory syncytial virus in adults age 60 and older
Lire la suite »
 Health Canada approves Moderna's mRNA RSV vaccine, company saysTORONTO — Moderna says Health Canada has approved its mRNA vaccine to protect against respiratory syncytial virus in adults age 60 and older. The company says it expects the RSV vaccine — called mRESVIA — to be available in early 2025.
Health Canada approves Moderna's mRNA RSV vaccine, company saysTORONTO — Moderna says Health Canada has approved its mRNA vaccine to protect against respiratory syncytial virus in adults age 60 and older. The company says it expects the RSV vaccine — called mRESVIA — to be available in early 2025.
Lire la suite »
 Health Canada approves Moderna's mRNA RSV vaccine, company saysTORONTO — Moderna says Health Canada has approved its mRNA vaccine to protect against respiratory syncytial virus in adults age 60 and older. The company says it expects the RSV vaccine — called mRESVIA — to be available in early 2025.
Health Canada approves Moderna's mRNA RSV vaccine, company saysTORONTO — Moderna says Health Canada has approved its mRNA vaccine to protect against respiratory syncytial virus in adults age 60 and older. The company says it expects the RSV vaccine — called mRESVIA — to be available in early 2025.
Lire la suite »
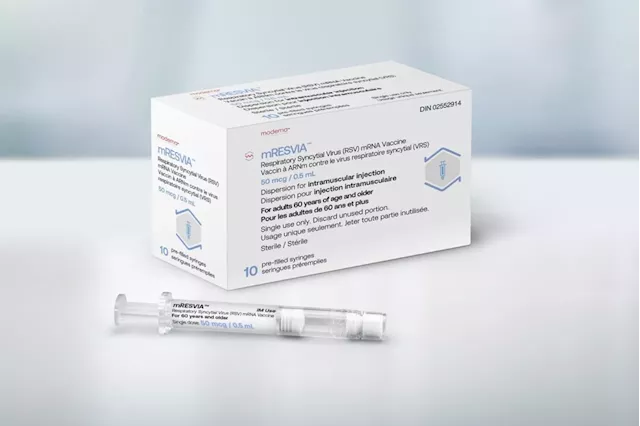 Health Canada approves Moderna's mRNA RSV vaccine, company saysTORONTO — Moderna says Health Canada has approved its mRNA vaccine to protect against respiratory syncytial virus in adults age 60 and older. The company says it expects the RSV vaccine — called mRESVIA — to be available in early 2025.
Health Canada approves Moderna's mRNA RSV vaccine, company saysTORONTO — Moderna says Health Canada has approved its mRNA vaccine to protect against respiratory syncytial virus in adults age 60 and older. The company says it expects the RSV vaccine — called mRESVIA — to be available in early 2025.
Lire la suite »