ABCNews
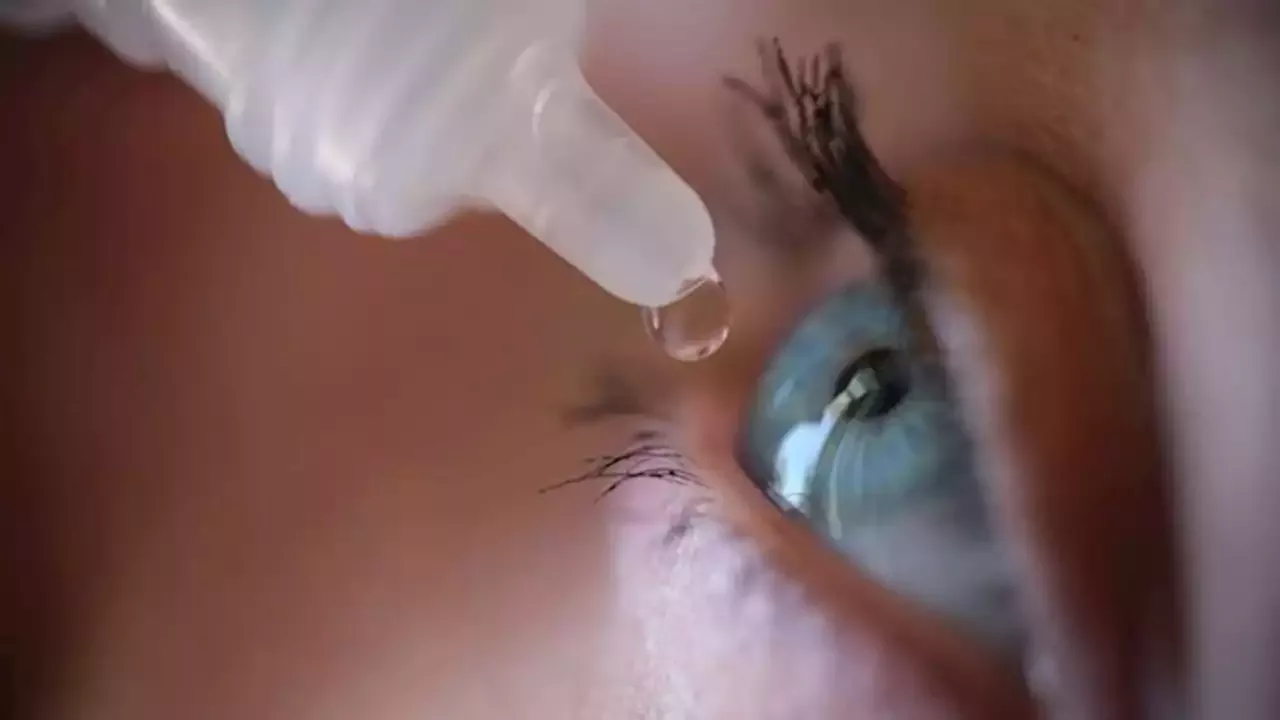
In their letters, the FDA also said people using these unapproved eye products that claim to treat or cure certain conditions may cause delay or stoppage of treatments that are approved by the agency. The FDA told the companies they have 15 days to respond to the FDA in writing to address any steps they have taken to correct violations.