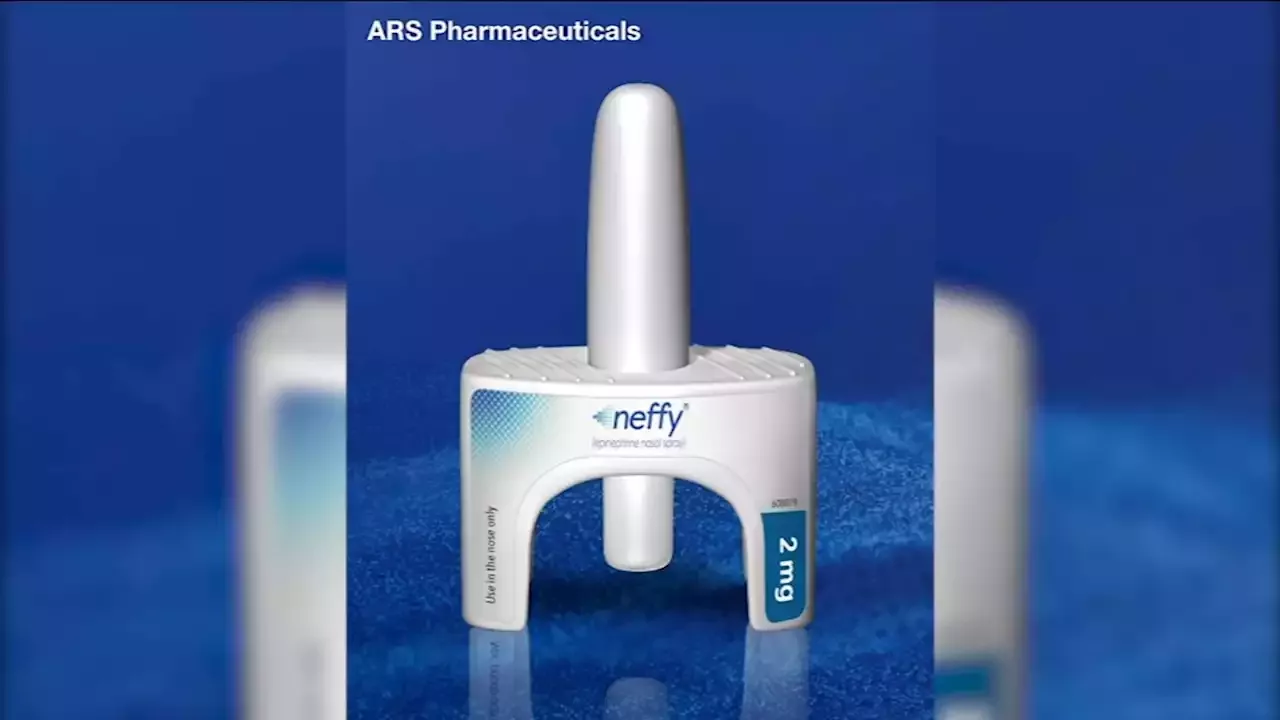
About 40 million people in the US experience extreme allergic reactions. If approved, Neffy would be the first needle-free nasal spray epinephrine treatment for people with severeRegulators at the U.S. Food and Drug Administration declined to approve a needle-free nasal spray equivalent to an EpiPen, pending further study, according to the company developing it.
ARS Pharma developed the product, Neffy, which is inhaled and would have been available by prescription if approved. Neffy would be a first-of-its-kind alternative to an EpiPen, which is commonly used to treat anaphylaxis, or severe allegoric reactions. The FDA said they haven't yet seen enough evidence to support approval. The company will have an opportunity to run additional studies and apply for approval again.
'We are very surprised by this action and the late requirement at this time to change the repeat-dose study from a post-marketing requirement, which we had previously aligned on with FDA, to a pre-approval requirement, particularly given the positive Advisory Committee vote," Richard Lowenthal, CEO of ARS, said in
Business Business Latest News, Business Business Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
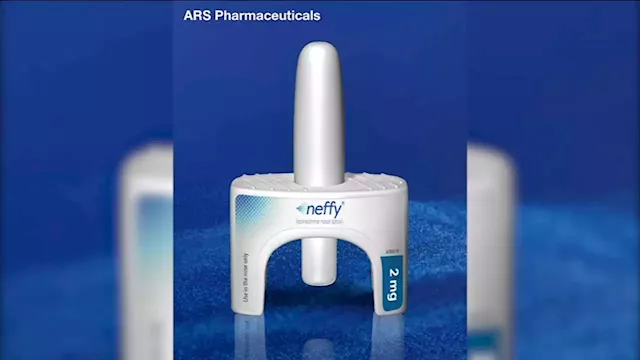 FDA rejects 'Neffy,' nasal spray alternative to EpiPen, company saysNeffy is inhaled and would have been available by prescription if approved.
FDA rejects 'Neffy,' nasal spray alternative to EpiPen, company saysNeffy is inhaled and would have been available by prescription if approved.
Read more »
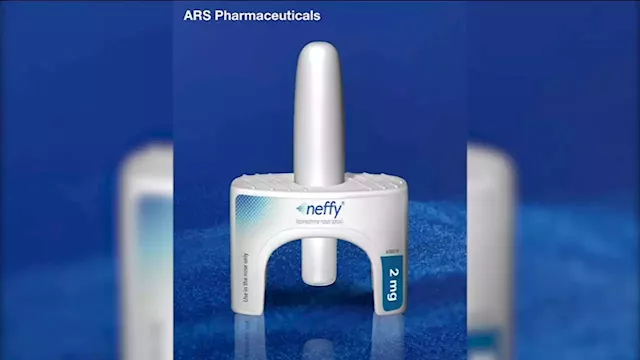 FDA rejects 'Neffy,' nasal spray alternative to EpiPen, company saysNeffy is inhaled and would have been available by prescription if approved.
FDA rejects 'Neffy,' nasal spray alternative to EpiPen, company saysNeffy is inhaled and would have been available by prescription if approved.
Read more »
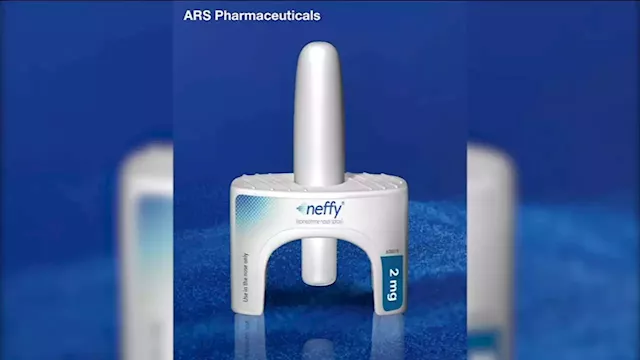 FDA rejects 'Neffy,' nasal spray alternative to EpiPen, company saysNeffy is inhaled and would have been available by prescription if approved.
FDA rejects 'Neffy,' nasal spray alternative to EpiPen, company saysNeffy is inhaled and would have been available by prescription if approved.
Read more »
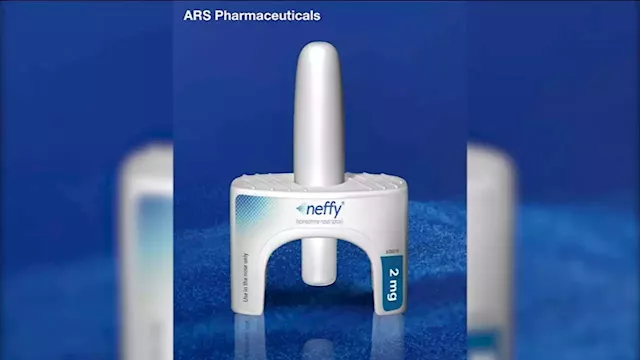 FDA rejects 'Neffy,' nasal spray alternative to EpiPen, company saysNeffy is inhaled and would have been available by prescription if approved.
FDA rejects 'Neffy,' nasal spray alternative to EpiPen, company saysNeffy is inhaled and would have been available by prescription if approved.
Read more »