The FDA typically nudges manufacturers to withdraw drugs voluntarily; it is rare for the agency to force a drug withdrawal.Updated April 6, 2023 10:46 am ET
The only drug on the market to help prevent preterm births must stop being sold immediately, a move that comes after scientists at the Food and Drug AdministrationThe FDA said Thursday it is withdrawing its approval for the drug Makena and its generics because the drugs no longer are shown to be effective and the benefits don’t outweigh the risks. Makena, as well as its generics, can no longer be lawfully distributed in interstate commerce.
Wat
If it doesn't work, it shouldn't be used.
Sverige Senaste nytt, Sverige Rubriker
Similar News:Du kan också läsa nyheter som liknar den här som vi har samlat in från andra nyhetskällor.
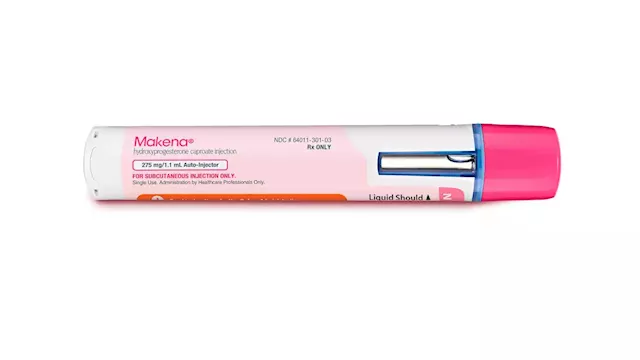 FDA forces unproven premature birth drug Makena off marketThe Food and Drug Administration is ordering an unproven drug intended to prevent premature births off the market
FDA forces unproven premature birth drug Makena off marketThe Food and Drug Administration is ordering an unproven drug intended to prevent premature births off the market
Källa: WOKVNews - 🏆 247. / 63 Läs mer »
